Who we are
- Who we are
- Mission and vision
- Principles of action
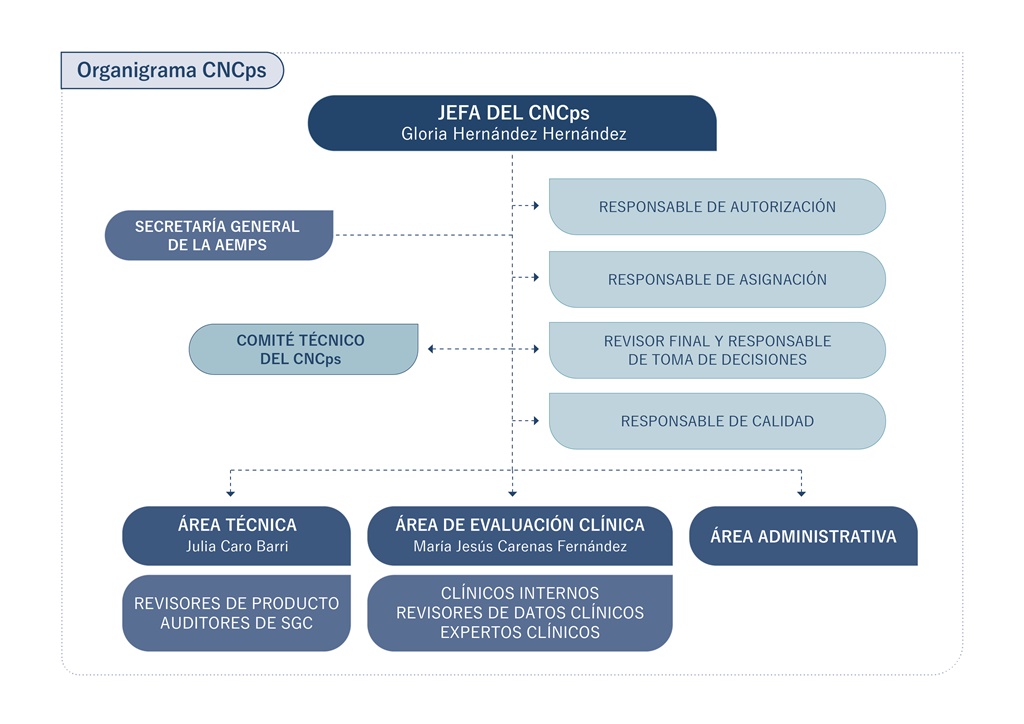
- Organigram
- Structure
- Charter of Services
- Work plans
- Annual activity reports
The CNCps is the National Center for the Certification of Medical Devices, a general sub-directorate attached to the Spanish Agency for Medicines and Medical Devices (AEMPS) that has the competence to act as a notified body and the certification of standards of quality systems specific to the medical device sector.
The more than 25 years of designation as a notified body attest to its technical competence in the evaluation of a wide variety of medical devices identified on the market by the number 0318 appearing next to the CE marking. Among others, it is worth mentioning implantable products, ophthalmic and ophthalmic products, reagents for the determination of infectious diseases and determination of blood groups, products for the administration of medicines and medicinal gases, products for sampling, active products for diagnosis, rehabilitation and motorization of patients, surgical instruments, products for wound care, sterile material, software for diagnosis or planning of treatments and surgical interventions.
In recent years, more than 1,700 audits have been carried out on national and international companies in the health products sector that have manufacturing technologies such as biotechnology, chemical, mechanical or thermal processing, applied to different metallic, plastic, textile or ceramic materials.
Mission
To provide confidence that the certifications issued and the medical devices that bear the CE 0318 mark are of quality, safe and effective, and contribute to the protection of the health of patients and users.
Vision
To be the reference body for the certification of quality products and systems in the field of medical devices, working with impartiality, independence, technical competence and vocation of public service.
The actions of the CNCps are carried out respecting the principles of independence and impartiality, confidentiality, responsibility and transparency.
The CNCps has established a risk-based system to promote and apply the principles of independence, impartiality, objectivity and lack of interests, throughout its structure, personnel and activities. This system encompasses all stakeholders and potential sources that threaten the preservation of these principles.
The staff of the CNCps are mostly civil servants and public employees who are obliged to respect the principles of objectivity, absence of interests and ability to respond on their actions (civil liability) established in Royal Legislative Decree 5/2015, of October 30, Basic Statute of the Public Employee. However, CNCps conducts conflict of interest assessment to all personnel involved in conformity assessment in accordance with our general procedure.
With the publication of the Charter of Services, the CNCps complies with theRoyal Decree 951/2005, of 29 July, establishing the general framework for quality improvement in the General Administration of the State, which integrates a set of programs including the one related to service letters. The aim of these instruments is to improve the quality of public services, to provide public authorities with consolidated information for decision-making and to promote transparency through information and public dissemination of the level of quality offered to users.
CNCps’ Charter of Services 2023-2026 has been approved through theResolution of 12 December 2022, by the Under-Secretary for Health, after a favourable report from the Directorate-General for Public Governance (Ministry of Finance and the Civil Service). This resolution has been published in the Official State Gazette, which gives an account of the approval of the letter and its availability to citizens.